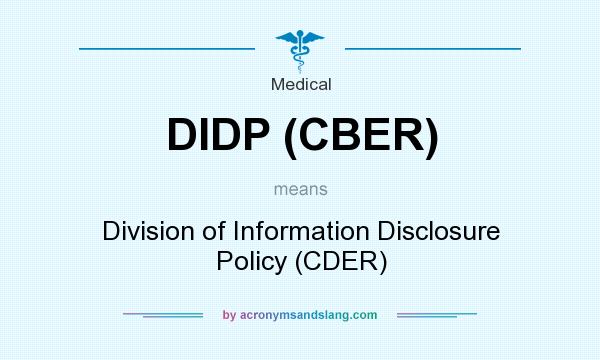
What does DIDP (CBER) mean?
DIDP (CBER) means Division of Information Disclosure Policy (CDER)
What is the abbreviation for Division of Information Disclosure Policy (CDER)?
Division of Information Disclosure Policy (CDER) can be abbreviated as DIDP (CBER)
|
|
Most popular questions people look for before coming to this page
| Q: A: |
What does DIDP (CBER) stand for? DIDP (CBER) stands for "Division of Information Disclosure Policy (CDER)". |
| Q: A: |
How to abbreviate "Division of Information Disclosure Policy (CDER)"? "Division of Information Disclosure Policy (CDER)" can be abbreviated as DIDP (CBER). |
| Q: A: |
What is the meaning of DIDP (CBER) abbreviation? The meaning of DIDP (CBER) abbreviation is "Division of Information Disclosure Policy (CDER)". |
| Q: A: |
What is DIDP (CBER) abbreviation? One of the definitions of DIDP (CBER) is "Division of Information Disclosure Policy (CDER)". |
| Q: A: |
What does DIDP (CBER) mean? DIDP (CBER) as abbreviation means "Division of Information Disclosure Policy (CDER)". |
| Q: A: |
What is shorthand of Division of Information Disclosure Policy (CDER)? The most common shorthand of "Division of Information Disclosure Policy (CDER)" is DIDP (CBER). |
Abbreviations or Slang with similar meaning
- DIDP - Division of Information Disclosure Policy
- DAPR (CDER) - Division of Applied Pharmacology Research (CDER)
- DAVP (CDER) - Division of Anti-viral Products (CDER)
- DBOP (CDER) - Division of Biologic Oncology Products (CDER)
- DCP 1 (CDER) - Division of Clinical Pharmacology 1 (CDER)
- DCP 2 (CDER) - Division of Clinical Pharmacology 2 (CDER)
- DCP 3 (CDER) - Division of Clinical Pharmacology 3 (CDER)
- DCP 4 (CDER) - Division of Clinical Pharmacology 4 (CDER)
- DCP 5 (CDER) - Division of Clinical Pharmacology 5 (CDER)
- DDOP (CDER) - Division of Drug Oncology Products (CDER)
- DDRE (CDER) - Division of Drug Risk Evaluation (CDER)
- DMB (CDER) - Division of Management and Budget (CDER)
- DMIP - Division of Medical Imaging Products (CDER)
- DNCE (CDER) - Division of Nonprescripton Clinical Evaluation (CDER)
- DNRD (CDER) - Division of Nonprescription Regulation Development (CDER)
- DODP (CDER) - Division of Oncologic Drug Products (CDER)
- DPDD (CDER) - Division of Pediatric Drug Development (CDER)
- DPE (CDER) - Division of Post-marketing Development (CDER)
- DPQR (CDER) - Division of Product Quality Research (CDER)
- DTD (CDER) - Division of Training and Development (CDER)